⬤ Takeda has announced positive Phase 3 results for zasocitinib, an oral TYK2 inhibitor for psoriasis that stands out as one of the most advanced AI-assisted drugs to reach late-stage clinical trials. Originally developed by Nimbus Therapeutics using artificial intelligence techniques, the therapy was later acquired by Takeda and has now successfully completed pivotal Phase 3 testing.
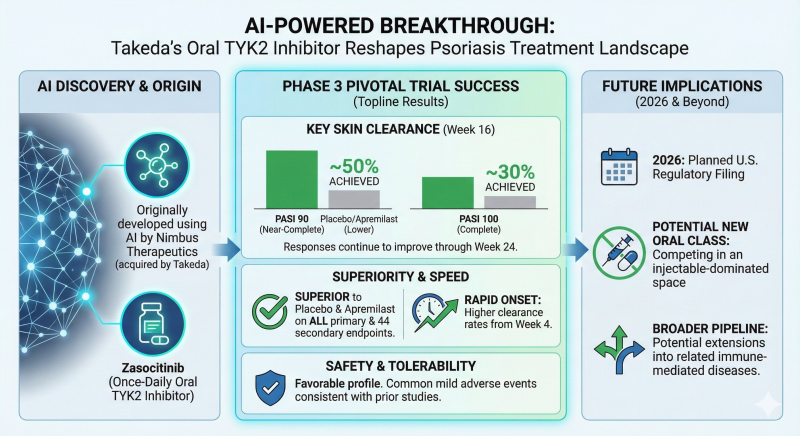
⬤ The trial met its primary endpoints with impressive skin clearance results. By Week 16, approximately 50% of patients achieved PASI 90—meaning near-complete skin clearance—while around 30% reached PASI 100, which represents total clearance. These outcomes beat both placebo and apremilast, a commonly prescribed oral treatment. Patient responses continued improving through Week 24, suggesting the drug's effects hold up over time.
⬤ The data showed quick action and consistent performance across the board. Higher clearance rates appeared as early as Week 4, and zasocitinib outperformed placebo and apremilast across all primary endpoints plus 44 secondary measures. The safety profile looked solid, with most side effects being mild and matching earlier study results. The once-daily oral format gives it an edge in a market where injectable biologics have been the go-to option.

⬤ Beyond psoriasis, this Phase 3 win opens bigger doors. Takeda plans to file for U.S. approval in 2026 and is eyeing expansion into other immune-related conditions. The success shows how AI is moving beyond early discovery into serious clinical development. For Takeda, it strengthens their immunology pipeline and signals how AI-driven drug development could reshape industry competition as more of these therapies push toward market.
 Alex Dudov
Alex Dudov
 Alex Dudov
Alex Dudov


